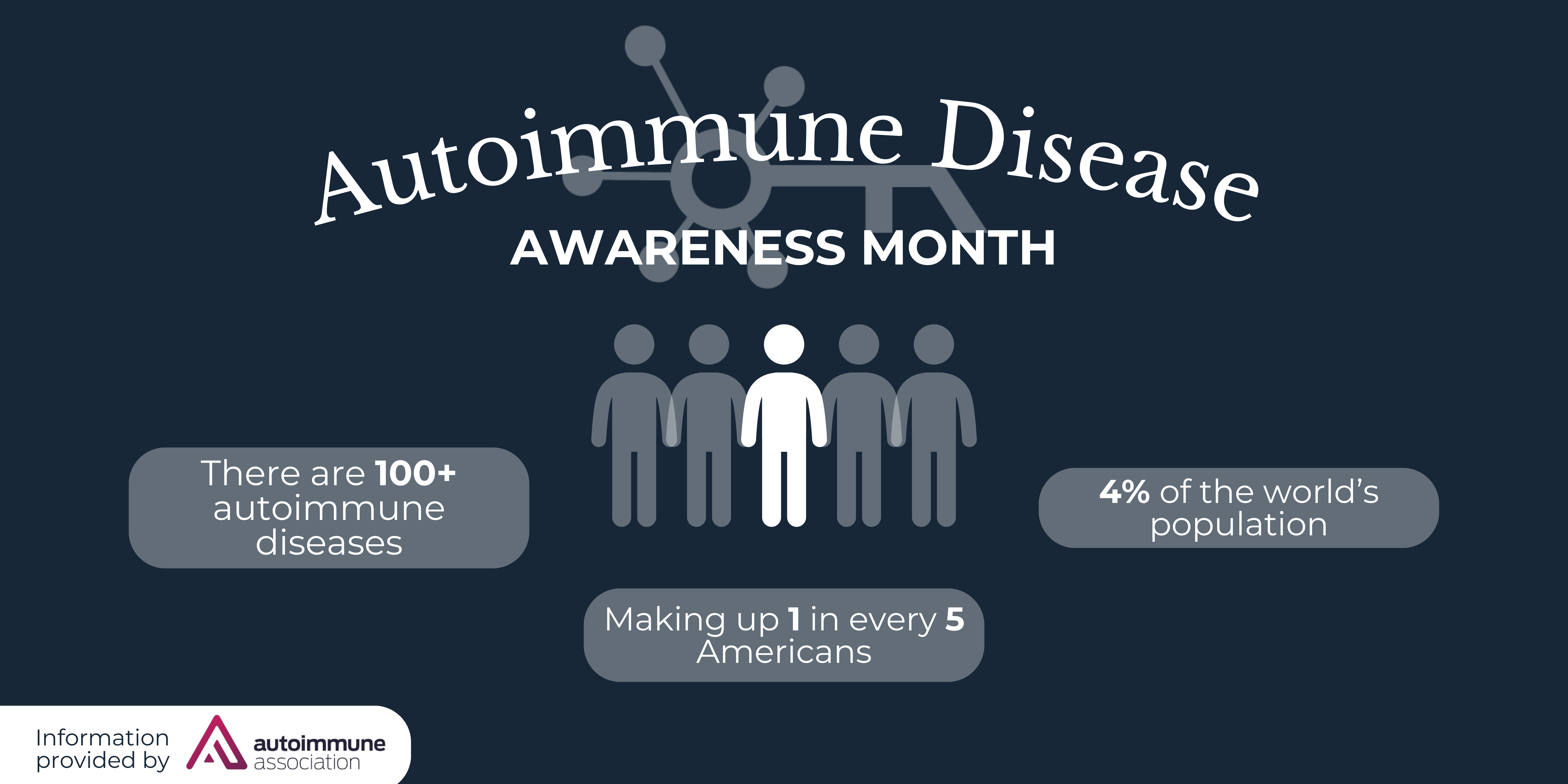
The end of the decade brought with it an exciting and critical trend for people living with rare diseases. Over the last eight years, the Center for Drug Evaluation and Research (CDER) approved more than twice as many orphan drugs as in the previous eight years (see graph below).
People living with rare diseases often face a long and difficult journey to diagnosis. During this time patients are going from doctor to doctor in search of answers, treatments, and hope that they are on the path the health. Too often patients go undiagnosed or, perhaps worse, are diagnosed and sadly informed that there is no treatment for their particular rare condition.
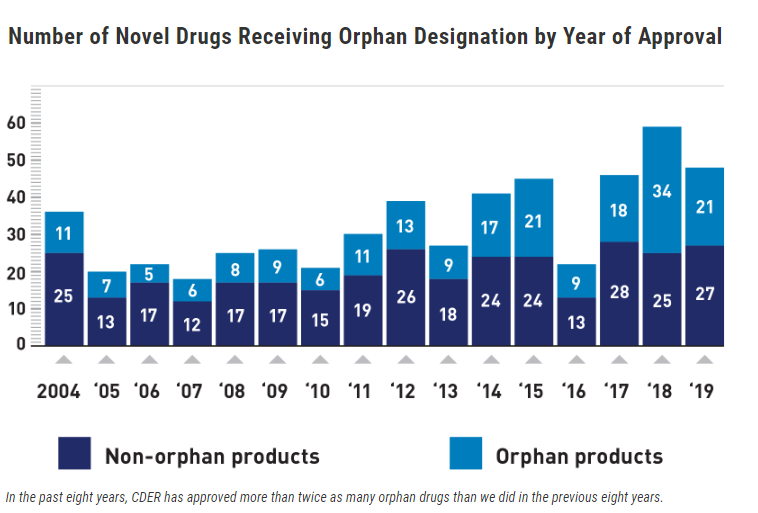
The number of annual orphan drug approvals are trending in favor of the rare disease community, but not as quickly as is necessary for the people suffering daily from the implications of their conditions. Every orphan drug approval is a scientific advancement and the epitome of hope for patients and their families who have waited years, maybe decades for a viable treatment option. Unfortunately, the majority of people living with rare diseases (roughly 95%) are still waiting for the creation and approval of drugs to treat their conditions. While every new drug launch inspires hope in the rare disease community, each approval is also a reminder of the many treatments yet to be discovered and many people who are not yet able to rely on tailored medications for their complex health needs.
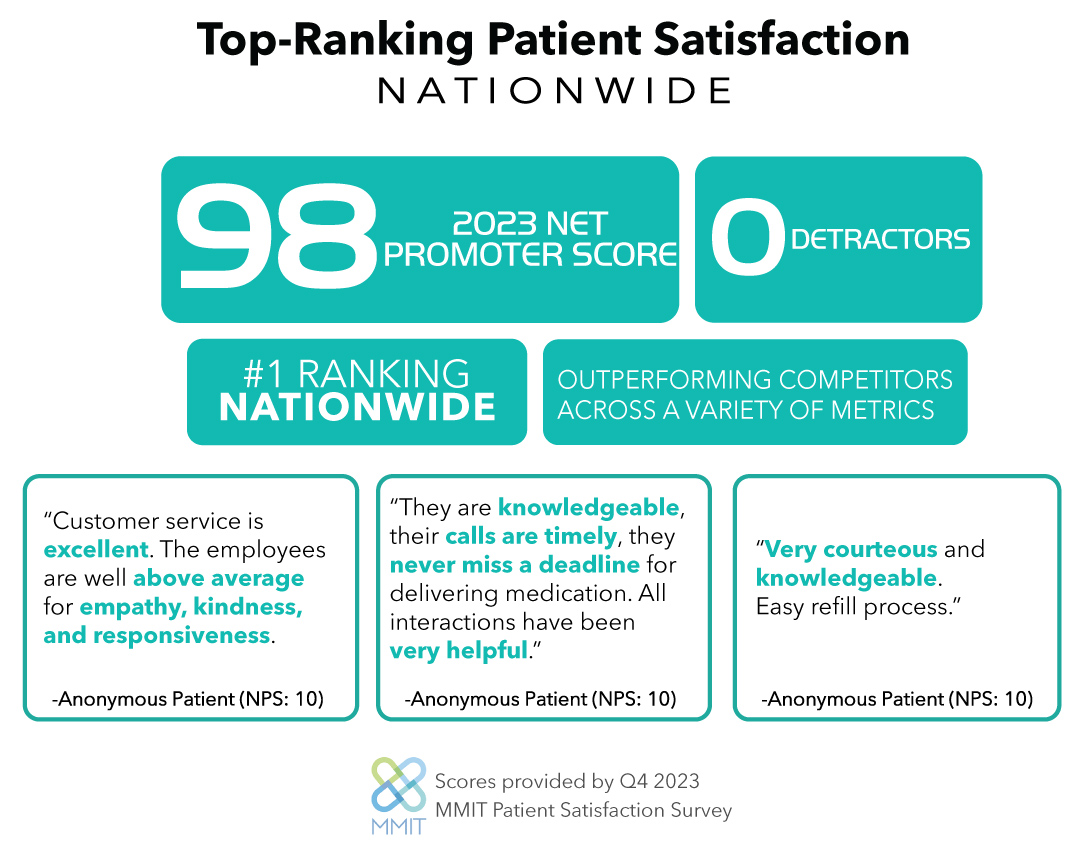
RareMed is proud to have made its mark on the industry since its inception. The company has grown rapidly as more and more orphan drug products have entered the market every year with significant HUB service needs. RareMed works closely with our industry partners to deliver tailored care to our patients, while collecting data and sharing detailed reports that help manufacturers gain insights into the patient populations that we serve. As we move into the next decade, RareMed maintains our commitment to accelerate care in rare for the benefit of people living with rare diseases who are hopeful for answers, treatments, and a better quality of life.