Read the Official RareMed Solutions Press Release.
Read the Official RareMed Solutions Press Release.
RareMed Solutions announces that it has been selected by PTC Therapeutics, Inc. to provide non-commercial dispensing services for the drug EMFLAZA® (deflazacort). RareMed will be the exclusive free drug provider for EMFLAZA®, managing the EMFLAZA® Start Program (ESP), Bridge, and Patient Assistance Programs. EMFLAZA® is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients two years of age or older.
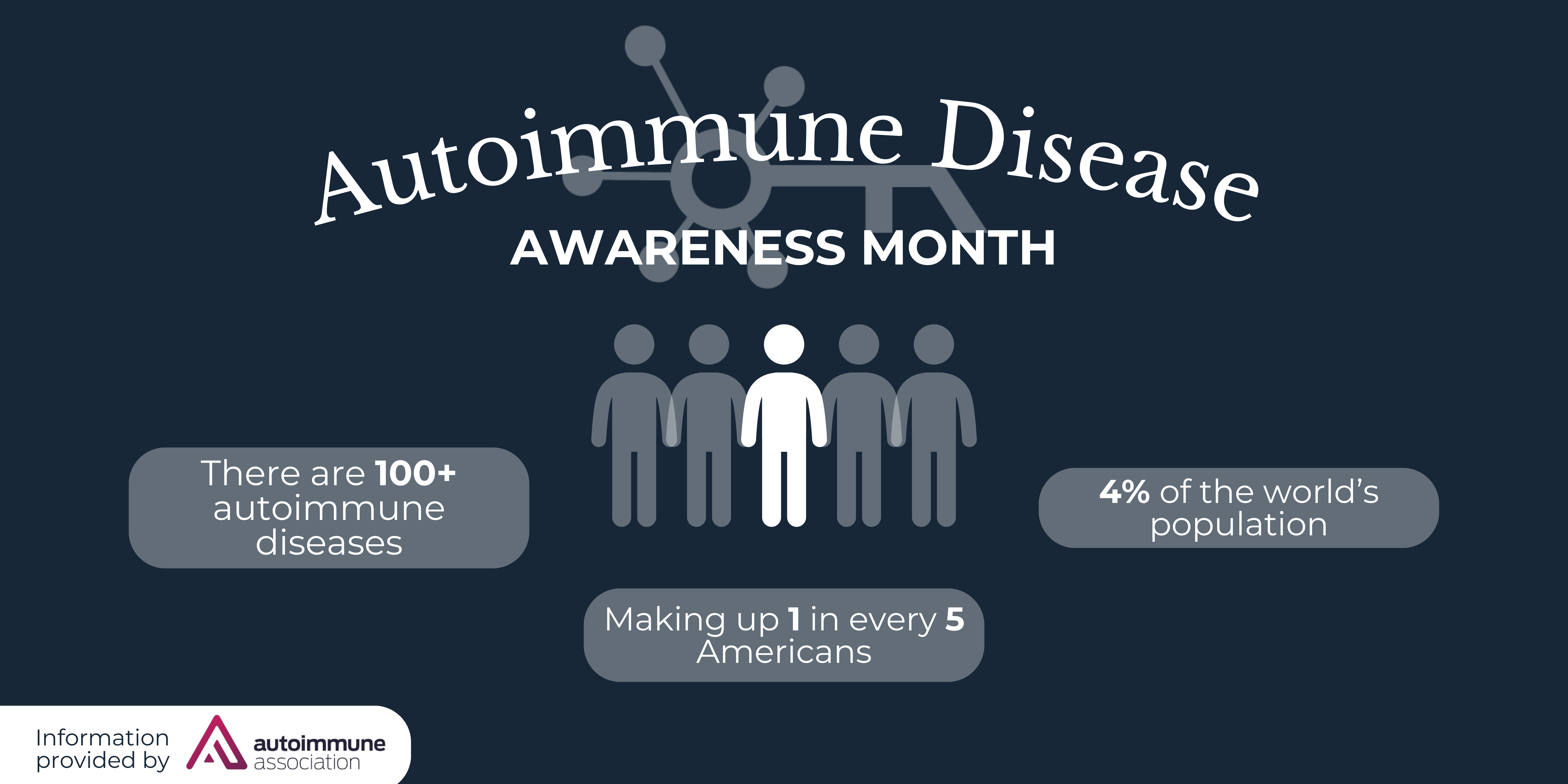
Primarily affecting males, DMD is a rare and fatal genetic disorder that results in progressive muscle weakness from early childhood and leads to premature death in the mid-twenties due to heart and respiratory failure. It is a progressive muscle disorder caused by the lack of functional dystrophin protein. Dystrophin is critical to the structural stability of skeletal, diaphragm, and heart muscles. Patients with Duchenne can lose the ability to walk as early as age ten, followed by loss of the use of their arms. Duchenne patients subsequently experience life-threatening lung complications, requiring the need for ventilation support, and heart complications in their late teens and twenties. More information regarding Duchenne is available through the Muscular Dystrophy Association and the Parent Project Muscular Dystrophy. Additionally, information and resources are available at www.duchenneandyou.com.
RareMed’s onsite non-commercial pharmacy will be providing EMFLAZA® to patients across the United States. RareMed’s RarePath™ technology will provide a personalized experience for our patients, healthcare providers, and network partners.
“RareMed is excited to partner with PTC Therapeutics, Inc, playing a central role in helping provide access to patients suffering from DMD. We look forward to serving the DMD community through our experienced and fully dedicated RareSupport™ team” said Dr. Gordon J. Vanscoy, RareMed’s Chairman and CEO.
About RareMed Solutions
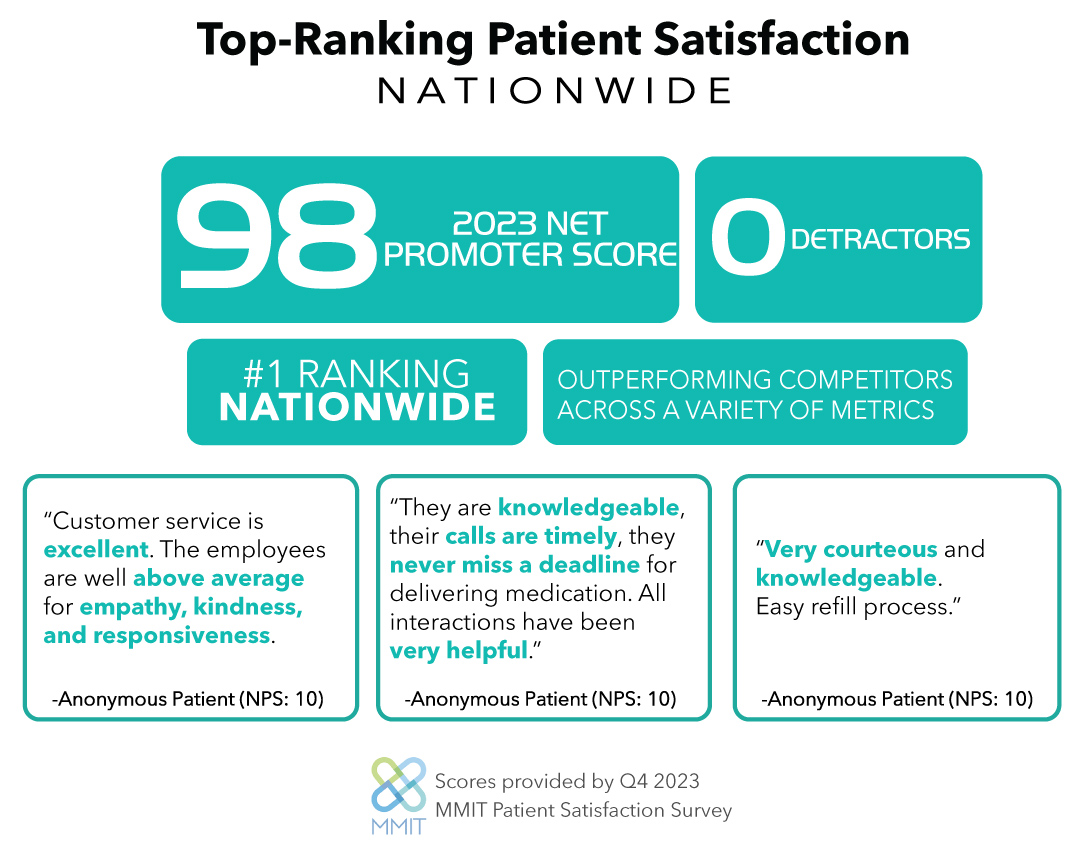
RareMed Solutions, LLC. is the nation’s only (pure) rare and devastating disorder patient service provider headquartered in a state-of-the-art facility in Pittsburgh, Pennsylvania. RareMed’s PSP services include non-commercial pharmacy dispensing, case management, co-pay, coupon, and financial assistance programs, reimbursement support, nursing support, healthcare professional education, and patient adherence & education. RareMed’s onsite non-commercial pharmacy is licensed to dispense in all states and the District of Columbia. The leadership team at RareMed has experience launching and managing complex therapies including injectables and products with cold-chain storage & shipment requirements. The company has a breadth of experience developing and maintaining therapy-specific solutions that ensure unparalleled manufacturer & patient satisfaction. RareMed’s undivided rare disease focus, highly trained associates, fully dedicated teams, and sophisticated proprietary technology enable it to meet the unique needs of its rare and devastating disorder biopharma partners.
To learn more about RareMed Solutions and the services we provide, visit our website https://www.raremed.com/services/
About PTC Therapeutics, Inc.
PTC Therapeutics, Inc. is a science-driven, global biopharmaceutical company focused on the discovery, development and commercialization of clinically differentiated medicines that provide benefits to patients with rare disorders. PTC Therapeutics Inc.’s ability to globally commercialize products is the foundation that drives investment in a robust and diversified pipeline of transformative medicines and our mission to provide access to best-in-class treatments for patients who have an unmet medical need.
For more information about EMFLAZA®, visit http://www.emflaza.com. If you are a prescribing physician, you can also download the enrollment form.
EMFLAZA® is a registered trademark of PTC Therapeutics, Inc.